Current issue
Online first
Archive
About the Journal
Aims and scope
Publisher and Editorial
Advertising policy
For Authors
Paper review procedures
Procedures protecting authentic authorship of papers
Paper preparation manual
Plagiarism check
Publication ethics
Reviewers
APC
Editorial and Scientific Board
Contact
Reviewers
Experimental and kinetic analysis of low to intermediate temperature auto-ignition of binary ethylene-acetylene blends
1
Division of Aerospace Engineering, Karunya Institute of Technology and Sciences, India
Submission date: 2026-01-25
Final revision date: 2026-02-06
Acceptance date: 2026-02-09
Online publication date: 2026-02-13
Corresponding author
Kesavan Marimuthu
Division of Aerospace Engineering, Karunya Institute of Technology and Sciences, India
Division of Aerospace Engineering, Karunya Institute of Technology and Sciences, India
KEYWORDS
TOPICS
ABSTRACT
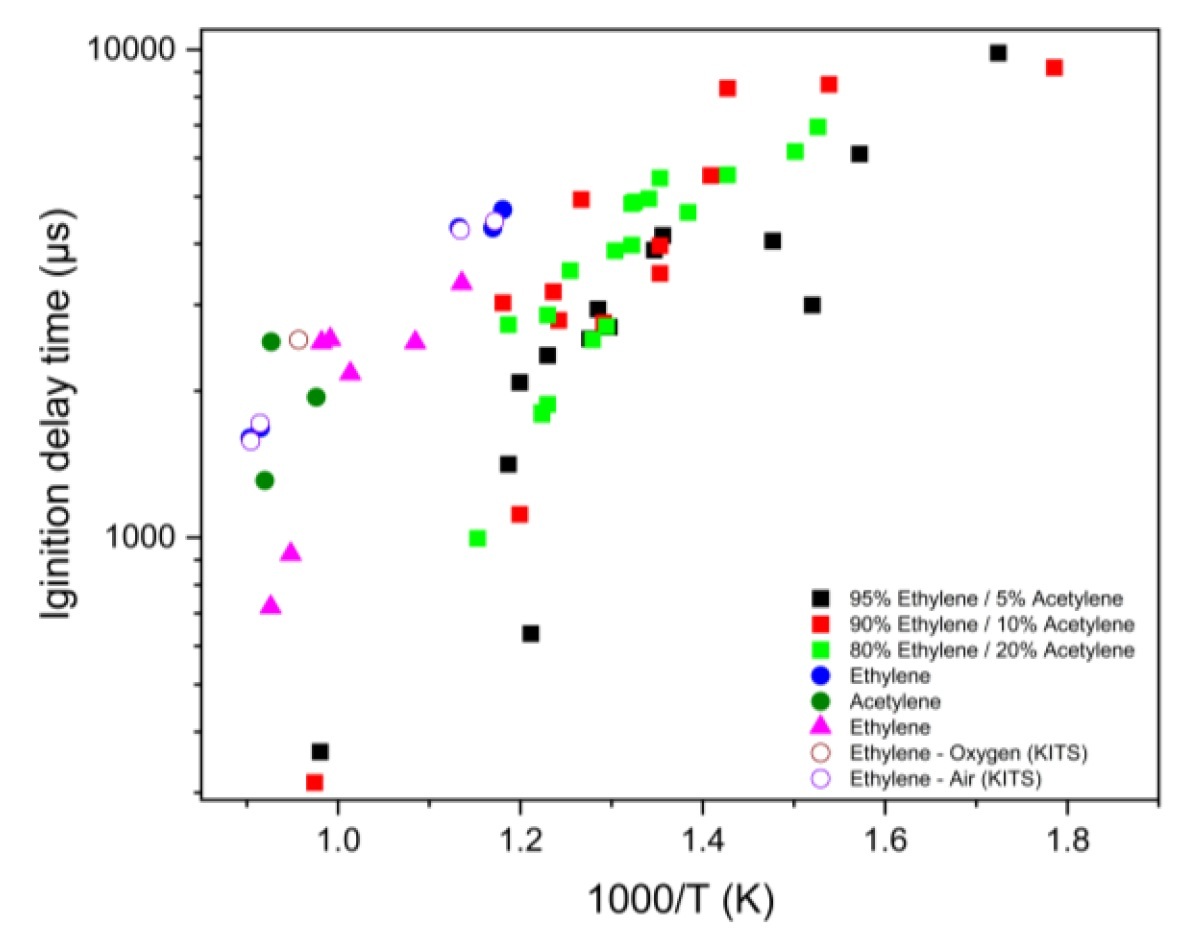
Understanding the ignition characteristics of binary hydrocarbon blends is essential for designing high-speed propulsion systems such as scramjets, where ignition under short residence time is a critical challenge. In this work, the ignition delay behaviour of ethylene-acetylene/air mixtures was examined through shock tube experiments and kinetic simulations under engine-relevant conditions. Ethylene was used as the primary fuel and blended with acetylene at 5%, 10%, and 20% by volume to form binary mixtures, at an equivalence ratio of 1.0, temperatures between 560–1030 K, and pressures of 2.5–9 bar. The ignition delay time was determined from peak pressure rise and CH* chemiluminescence behind the reflected shock. Unlike previous blended fuel studies dominated by saturated hydrocarbons, this work presents a comprehensive dataset for ethylene-acetylene blends at low to intermediate temperatures and increasing the acetylene fraction from 5% to 20% reduces the ignition delay time by up to 50–60% in the 700–850 K and 2–5 bar regime. Numerical simulations were performed using ANSYS Chemkin in a closed, homogeneous, constant-volume reactor with the NUIG, ARAMCO, LLNL, and San Diego mechanisms. The sensitivity and rate-of-production analyses reveal that ignition is governed by HO2–H2O2 radical chemistry, with the thermal decomposition of H2O2 triggering rapid OH formation. Acetylene enhances ignition by promoting the regeneration of HCCO radicals and accelerating the transition to chain-branching chemistry.
REFERENCES (35)
1.
Baigmohammadi M, Patel V, Nagaraja S, Ramalingam A, Martinez S, Panigrahy S. Comprehensive experimental and simulation study of the ignition delay time characteristics of binary blended methane, ethane, and ethylene over a wide range of temperature, pressure, equivalence ratio, and dilution. Energy Fuels. 2020;34(7):8808-8823. https://doi.org/10.1021/acs.en....
2.
Baigmohammadi M, Patel V, Martinez S, Panigrahy S, Ramalingam A, Burke U. A comprehensive experimental and simulation study of ignition delay time characteristics of single fuel C1-C2 hydrocarbons over a wide range of temperatures, pressures, equivalence ratios, and dilutions. Energy Fuels. 2020;34(3):3755-3771. https://doi.org/10.1021/acs.en....
3.
Boruc ŁJ, Kapusta J, Kindracki J. Selection of the method for determination of ignition delay of hypergolic propellants. Combustion Engines. 2024;199(4):104-111. https://doi.org/10.19206/CE-19....
4.
Burcat A, Scheller K, Lifshitz A. Shock-tube investigation of comparative ignition delay times for C1-C5 alkanes. Combust Flame. 1971;16(1):29-33. https://doi.org/10.1016/S0010-....
5.
Chemical-Kinetic Mechanisms for Combustion Applications. San Diego Mechanism Web Page. University of California San Diego. Available from: http://combustion.ucsd.edu.
6.
Dagaut P, Boettner JC, Cathonnet M. Ethylene pyrolysis and oxidation: A kinetic modeling study. Int J Chem Kinet. 1990;22(6):641-664. https://doi.org/10.1002/kin.55....
7.
Dagaut P, Cathonnet M, Boettner J. Kinetics of ethane oxidation. Int J Chem Kinet. 1991;23(5):437-455. https://doi.org/10.1002/kin.55....
8.
De Vries J, Hall JM, Simmons SL, Rickard MJA, Kalitan DM. Ethane ignition and oxidation behind reflected shock waves. Combust Flame. 2007;150:137-150. https://doi.org/10.1016/j.comb....
9.
De Vries J, Petersen EL. Autoignition of methane-based fuel blends under gas turbine conditions. Proc Combust Inst. 2007;31(2):3163-3171. https://doi.org/10.1016/j.proc....
10.
Gallagher SM, Curran HJ, Metcalfe WK, Healy D, Simmie JM, Bourque G. Rapid compression machine study of propane oxidation in the negative temperature coefficient regime. Combust Flame. 2008;153(1-2):316-333. https://doi.org/10.1016/j.comb....
11.
Herzler J, Jerig L, Roth P. Shock-tube study of the ignition of propane at intermediate temperatures and high pressures. Combust Sci Technol. 2004;176(10):1627-1637. https://doi.org/10.1080/001022....
12.
Holton M, Gokulakrishnan P, Klassen MS, Roby RJ, Jackson GS. Autoignition delay time measurements of methane, ethane, and propane pure fuels and methane-based fuel blends. J Eng Gas Turbines Power. 2010;132(9):1-9. https://doi.org/10.1115/1.4000....
13.
Huang J, Li F, Qi Y. Comparative study on the impact of ethane/ethylene/acetylene addition on ignition of fuel-rich n-decane/air flame. Bull Ser B. 2020;82(4):185-198.
14.
Jach I, Cieślak I, Teodorczyk A. Investigation of glycerol doping on ignition delay times and laminar burning velocities of gasoline and diesel fuel. Combustion Engines. 2017;169(2):167-175. https://doi.org/10.19206/ce-20....
15.
Kesavan M, Sundararaj AJ, Williams M, Konda S, Kumar W. Experimental investigation of auto ignition of ethylene and propulsion grade kerosene. Russ J Phys Chem B. 2025;19(6):1338-1352. https://doi.org/10.1134/S19907....
16.
Kopp M, Donato NS, Petersen EL, Metcalfe WK, Burke SM, Curran HJ. Oxidation of ethylene-air mixtures at elevated pressures, part 1: experimental results. J Propuls Power. 2014;30(3):790-798. https://doi.org/10.2514/1.B348....
17.
Leschevich VV, Martynenko VV, Penyazkov OG, Sevrouk KL, Shabunya SI. Auto-ignitions of a methane/air mixture at high and intermediate temperatures. Shock Waves. 2016;26(5):657-672. https://doi.org/10.1007/s00193....
18.
Lowry W, De Vries J, Krejci M, Petersen E, Serinyel Z, Metcalfe W. Laminar flame speed measurements and modeling of pure alkanes and alkane blends at elevated pressures. J Eng Gas Turbines Power. 2011;133(9):1-9. https://doi.org/10.1115/1.4002....
19.
Lyle JL, Guna KR, Kumar P, Sundararaj AJ. Rupture dynamics of shock-tube diaphragm. Proc Int Conf Recent Adv Aerosp Eng (ICRAAE). 2017:1-4. https://doi.org/10.1109/ICRAAE....
20.
Marinov NM, Pitz WJ, Westbrook CK, Vincitore AM, Castaldi MJ. Aromatic and polycyclic aromatic hydrocarbon formation in a laminar premixed n-butane flame. Combust Flame. 1998;114(1-2):192-213. https://doi.org/10.1016/S0010-....
21.
Martinez S, Patel V, Panigrahy S, Sahu A, Nagaraja B. Experimental and kinetic modeling study of ignition delay characteristics of ethane/propane and ethylene/propane blends. Combust Flame. 2021;228:401-414. https://doi.org/10.1016/j.comb....
22.
Rose MS, Sundararaj AJ, Kumar S. Investigation of ignition delay of hydrocarbon fuel using shock tube. Int J Pure Appl Math. 2018;118(20):3687-3692.
23.
Sander R, Longwic R, Tarkowski S. Analysis of the influence of the n-hexane content in the mixture with rapeseed oil on the auto-ignition delay angle of the fuel. Combustion Engines. https://doi.org/10.19206/CE-21....
24.
Saxena S, Kahandawala MSP. Shock tube study of ignition delay in the combustion of ethylene. Combust Flame. 2011;158(6):1019-1031. https://doi.org/10.1016/j.comb....
25.
Shao J, Davidson DF. Shock tube study of ignition delay times in diluted methane, ethylene, propene and their blends at elevated pressures. Fuel. 2018;225:370-380. https://doi.org/10.1016/j.fuel....
26.
Song C, Saggese C, Kang D, Goldsborough SS, Wagnon SW, Kukkadapu G. Autoignition and heat release of gasoline surrogates and ethanol blends at engine-relevant conditions. Combust Flame. 2021;228:57-77. https://doi.org/10.1016/j.comb....
27.
Sundararaj AJ, Pillai BC, Subash AN, Haran AP, Kumar P. Investigation of ignition delay for low molecular weight hydrocarbon fuel using shock tube in reflected shock mode. J Geol Soc India. 2019;93:218-220. https://doi.org/10.1007/s12594....
28.
Sundararaj AJ, Pillai BC, Guna KR. Effect of temperature on ignition behaviour of seeded refined kerosene. Thermochim Acta. 2020;683:178469. https://doi.org/10.1016/j.tca.....
29.
Sundararaj AJ, Guna KR, William M. Effect of temperature on ignition of modified kerosene. Int J Engine Res. 2022;23(3):460-468. https://doi.org/10.1177/146808....
30.
Sundararaj AJ, Jose D, Sunny A, John ST, Kumar N, Gopalsamy. Effect of blast pressure on discrete models using a shock tube. Proc Int Conf Recent Adv Aerosp Eng (ICRAAE). 2017:1-4. https://doi.org/10.1109/ICRAAE....
31.
Walker BC. Shock-tube investigation of ignition delay times of blends of methane and ethane with oxygen. Aerosp Eng Commons. 2007. https://stars.library.ucf.edu/....
32.
Wan Z, Zheng Z, Wang Y, Zhang D, Li P, Zhang C. Shock tube study of ethylene/air ignition characteristics over a wide temperature range. Combust Sci Technol. 2020;192(12):2297-2305. https://doi.org/10.1080/001022....
33.
Wang H, Liu Y, Weng J, Glarborg P, Tian Z. New insights in low-temperature oxidation of acetylene. Proc Combust Inst. 2017;36(1):355-363. https://doi.org/10.1016/j.proc....
34.
Wang H, Dames E, Sirjean B, Sheen DA, Tango R, Violi A. High-temperature chemical kinetic model of n-alkanes and cycloalkanes oxidation (JetSurF). Available from: http://web.stanford.edu/group/....
35.
Zhou CW, Li Y, Burke U, Banyon C, Somers KP. Experimental and kinetic modeling study of 1,3-butadiene combustion: ignition delay time and laminar flame speed measurements. Combust Flame. 2018;197:423-438. https://doi.org/10.1016/j.comb....
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.